The Effects of Aeration on the Protein Expression of Fluorescent-Tagged Yeast Cells
In micro-fluid devices, the thermoplastic material consumption is tremendously increased compared to silicon or glass. Known in literature as the main material in the production of bio-reactor, Polidimentilsilicsan (PDMS) has disadvantages such as low endurance, sensitivity respect to chemicals, and permeability to the water stream. Yet, some thermoplastic materials such as COP, PMMA, and PS are more long-lasting and less expensive. In this project, It is aimed to produce a micro-fluid device (nano bio-reactor) by using COP, PMMA, and PS materials. In the production process, photolithography, grinding, warm roughening, and hot compressing procedures are followed. The suitability of yeast production within Lab-on-a-chip (LOC) devices made by these different materials is investigated based on the advantages of COP, PS, and PMMA are offered. The best-resulted material is used in the investigation of the effects of aeration on the protein expression of fluorescent-tagged yeast cells. After the ensued experiment on the LOC devices, COP material is responded the best as among others by having the least autofluorescent expression and the biggest growth in yeast cells. Correspondingly, the protein expression of fluorescent-tagged yeast cells is expecting to decrease due to the scarcity of aeration; after a while, it is increasing as it is expected. Outcomes of this research promise to uncover new aspects of the design of bio-reactors and micro-aeration at the nanoscale.
- First and foremost, 250 ml and 100 ml of Erlenmeyer flasks and 250 ml and 500 ml of Scott bottles are sterilized at 121°C, 15 minutes.
- Scott bottles are used for the preparation of medium. 4 gr of glucose, 60 ml of pure water are added to 250 ml of Scott, then it is sterilized at 121°C, 3 minutes.
- To the 500 ml of Scott, 0.34 gr of the yeast nitrogen base, 1 gr of ammonium sulfate, 0.02 gr of L-leucine, 0.004 gr of histidine, 0.006 gr of lysine, 0.004 gr of uracil, and 140 ml of water are added, then it is sterilized at 121°C for 15 minutes.
- It took approximately 3 hours to complete the medium for yeast.
- Saccharomyces Cerevisiae yeast cells are used for a 1:5 scale in Erlenmeyer flasks.
- Nop56 red-fluorescent tagged protein cells were used in the sample.
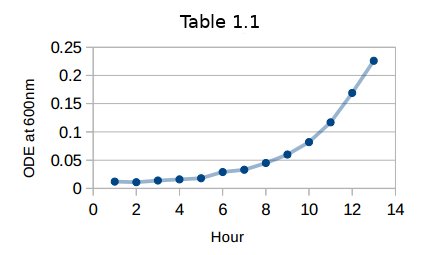
- Each hour wavelength dependence is measured by UV-Vis spectrometer. Recorded data is shown in Table 1.1
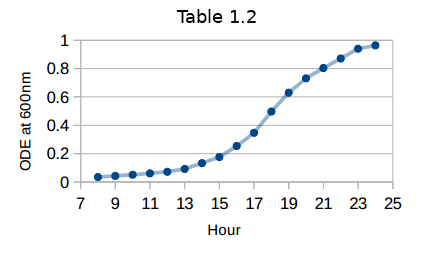
- In order to obtain a constant growth model, after 8 hours of the experimental data is shown in Table 1.2
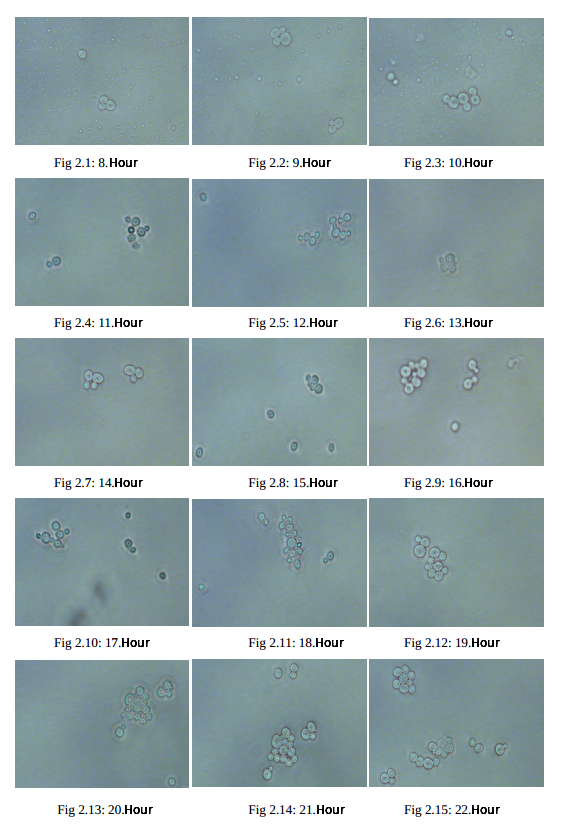
Measuring cell fluorescence using ImageJ
The level of cellular fluorescence from fluorescence microscopy images is determined in the ImageJ program. Cellular area, mean grey value, integrated density, and perimeter of the cell are measured by meticulous selection in each microscopy images. In addition, the same selection is repeated for the no fluoresce (background area) for the calculation of corrected total cell fluorescence.
P.S. The size of the background area is not important, 2 selections from around the cell are taken for accuracy.
Corrected total cell fluorescence (CTCF) is calculated based on the following formula:
CTCF = Integrated Density – (Area of selected cell X Mean fluorescence of background readings)
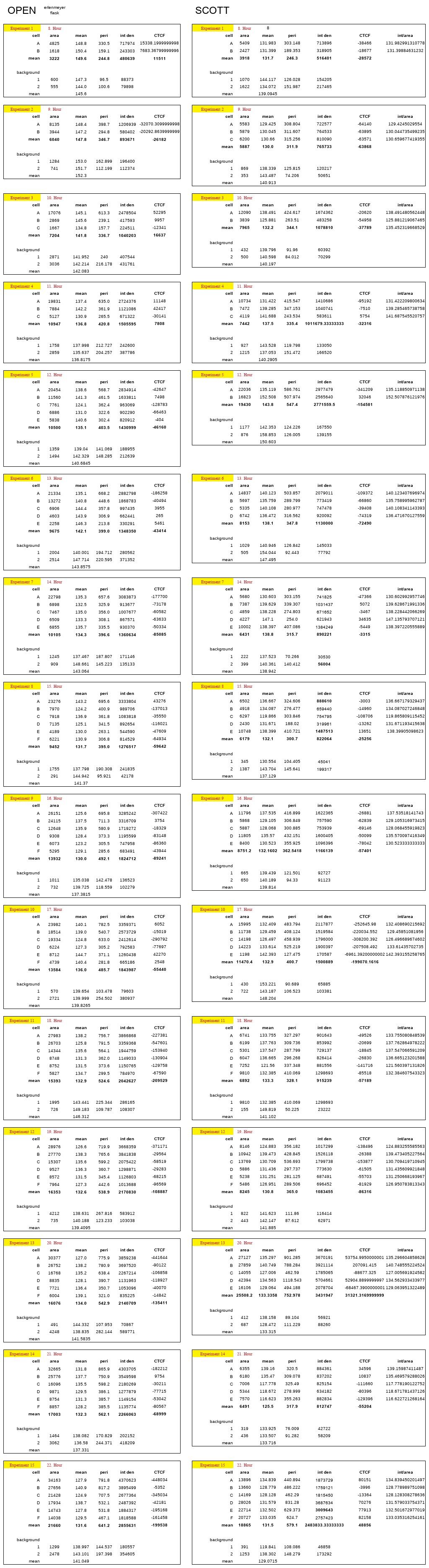
Mainly, two different analysis is made in order to examine the effects of aeration. First, the Erlenmyaer flasks are used for the experiment. Then, Scott bottles are used for isolation.
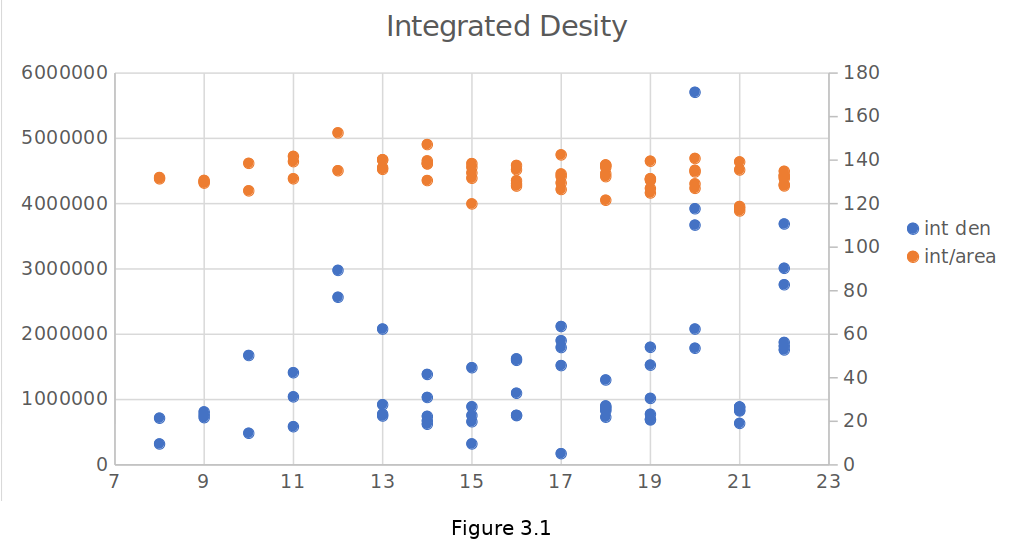
To see the accuracy of the data integrated density versus integrated density divided by area is shown in Figure 3.1
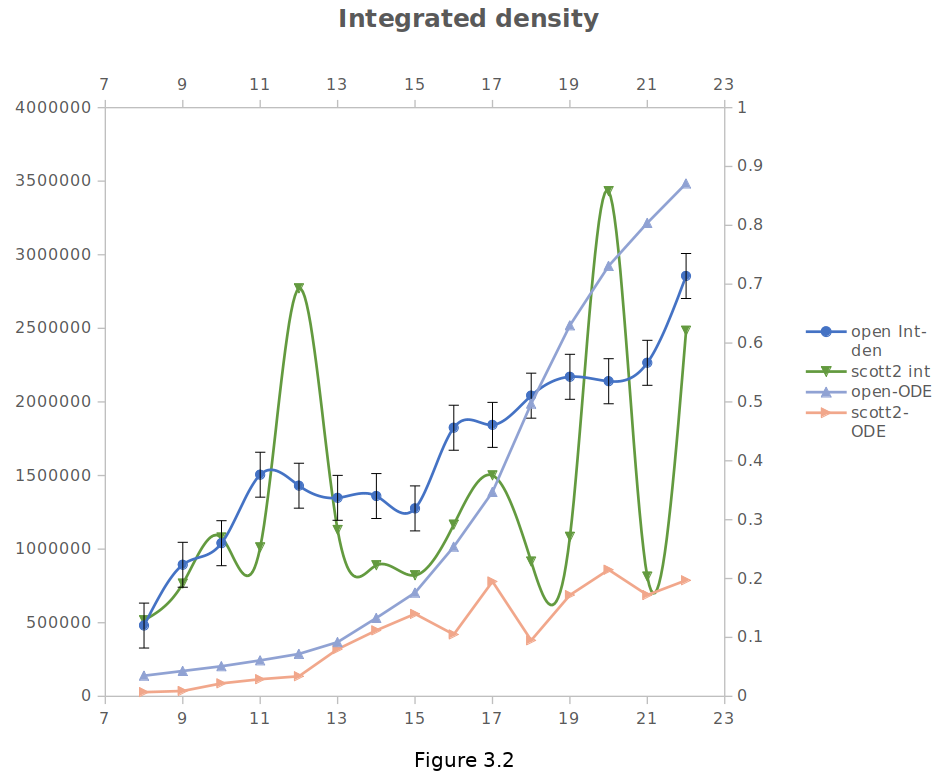
Moreover, to observe the actual pattern of growth in cells, both ODE and integrated density curvatures are shown in Figure 3.2
Data is proved that the protein expression of fluorescent-tagged yeast cells is decreasing due to the scarcity of aeration; after a while, it is increasing with somewhat a minor error.